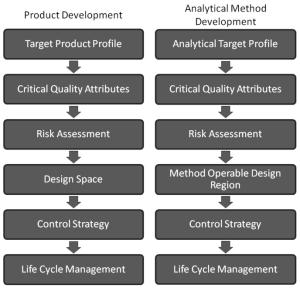
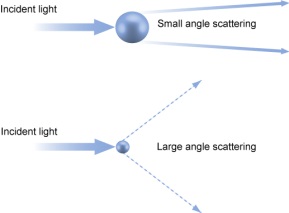
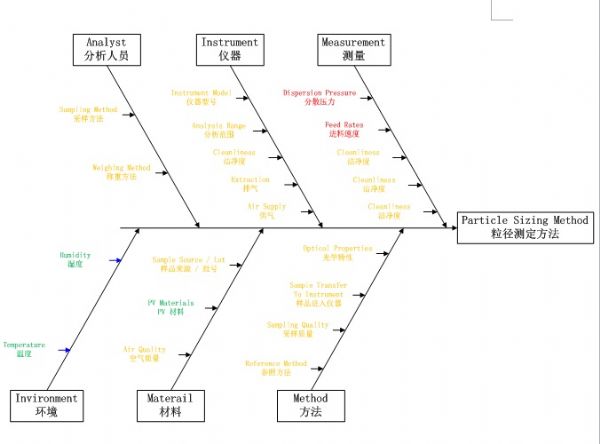
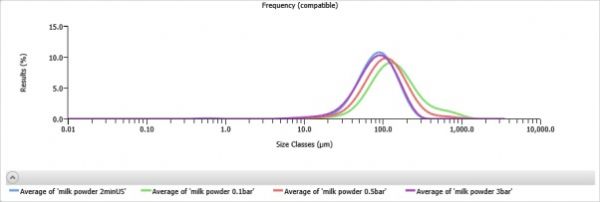
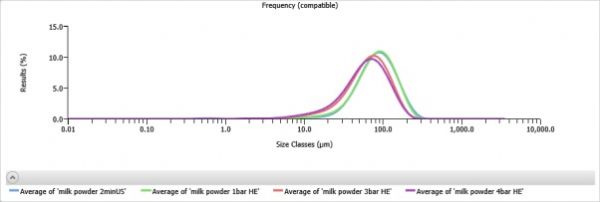
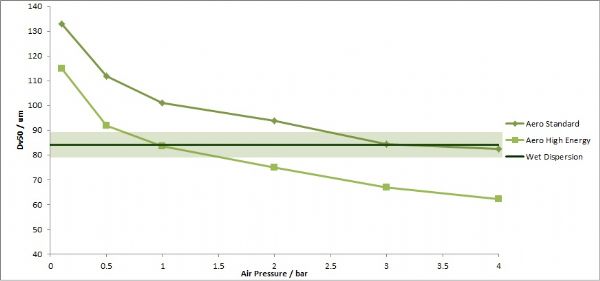
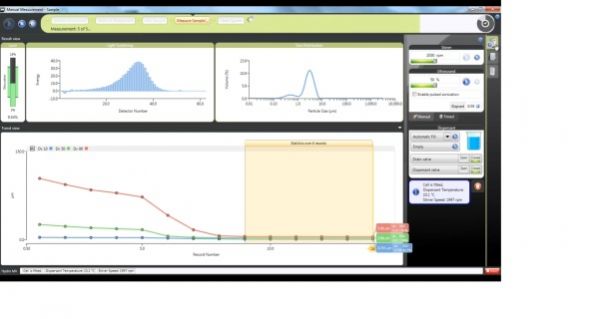
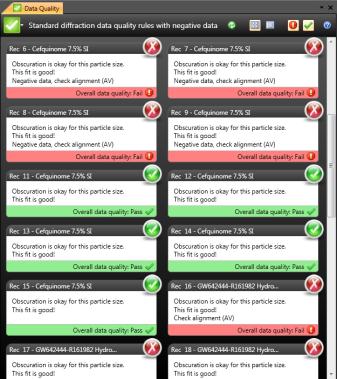
As the concepts and technologies embodied in QbD (quality comes from design) gradually become the second basic principle of the pharmaceutical industry, its application fields are also expanding. The development of analytical methods is currently the focus of QbD. The development, validation, and implementation of analytical methods is very similar to product development and can benefit from the systematic scientific approach that QbD values. Since drug development and manufacturing are inseparable from reliable analytical data, there is an urgent need to be more rigorous in the development of analytical methods. A new QbD method, namely analyzing QbD (AQbD), is gradually regarded as the future development direction. The goal of analytical method development is to develop, validate, and implement a method that provides the accurate and necessary analysis of the information required and, in any case, achieves the above objectives. First, we need to clarify the exact cause of the measurement, which is the same as the first step in the regular QbD to clarify the clinical performance of the product. Once this is determined, this process will help us develop awareness and understanding in order to control all aspects of the key elements that determine the performance of the analysis in the assessment method. This can truly reflect the working model of QbD in the full design space. This article will introduce AQbD and examine the terminology and methods that are constantly derived around it. We will use laser diffraction particle size analysis as a model to study the practical steps of applying AQbD, and will explore the characteristics of the instrument, in order to be able to choose to use this relatively new method. Introduction to QbD principle In order to better explore AQbD, we need to first review the widely accepted QbD definition. The definition of QbD first appeared in the International Drug Registration Coordination Meeting document Q8 (R2), and its interpretation of QbD is as follows: QbD is a systematic approach to development based on sound science and quality risk management, designed to pre-determine goals and emphasize product and process awareness and process control. Figure 1: The QbD workflow for product development, and a similar analytical method development workflow. product development Analytical method development Target product overview Analysis of target profiles Key quality characteristics Key quality characteristics Risk assessment Risk assessment Design space Methodable Design range Control Strategy Control Strategy Full lifecycle management Full lifecycle management The QbD workflow shown in Figure 1 clearly demonstrates this systematic approach. First, we need to determine the quality profile of the target drug (QTPP) and identify what products should be delivered. Second, we need to identify the various variables that must be controlled to achieve product performance, and the best way to implement control. First we need to determine performance, including key quality parameters (CQAs), key process parameters (CPPs), and key material properties (CMAs). Second, we should define the design space. The design space is the scope of the process, and it contains the exact range of CPP and CMAs to ensure that CQA can be implemented steadily. The final step is to determine the required control strategy to limit the job to the designated design space, and the control strategy runs through the process of improving the life cycle of the drug. In fact, one of the highlights of QbD is its ability to continuously optimize within the design space without further regulatory approval. AQbD: Introducing QbD into analytical method development The FDA's guidance on AQbD applications [1] highlights the potential benefits of this approach. The view is that AQbD can develop a robust approach to the entire product lifecycle. As with QbD, being able to comply with AQbD is associated with a degree of regulatory flexibility, allowing you to change the parameters of a method's design space. This is called the method's operational design interval (MODR). The first step in performing AQbD is to analyze the target profile (ATP), which is very similar to QTPP (Figure 1). ATP defines the goal of the analytical method development process, and the results obtained by this method are related to the overall QTPP. Determining the reason, use, and time of use of certain analytical information can help develop ATP. Additional goals for performance characteristics, such as accuracy and repeatability, stem from a more detailed analysis of these requirements. Next we need to determine a suitable analytical technique with reference to the requirements defined in the ATP. Once this technology is identified, AQbD will focus on method development, including risk assessments from fluctuations in associated factors: Analytical method Instrument configuration and maintenance Measurement parameter Material properties Environmental conditions This assessment determines the parameter values ​​that affect ATP, ie CQAs. Subsequently, we defined the MODR through a method of experimental design (DOE). This is the scope of CQAs' work and provides continuous support for the goals set by ATP. After completing the definition of MODR, we can implement reasonable method control and method validation according to the guidance in ICH Q2. Like QbD, AQbD works on the principle of identifying and processing variables to understand its potential impact. By identifying and analyzing the design space rather than applying a fixed set of measurement conditions, AQbD provides a responsive approach to the inherent changes encountered in everyday analysis throughout the life of a pharmaceutical product. This analytical method is not only effective in everyday use, but also greatly reduces potential failure risks when the method moves from research laboratories to actual quality control applications. The root cause of method conversion failure can often be traced back to the inconsistency of the characteristics of the normal operating environment and the inability to effectively capture and transmit information that ensures that the measurement is valid. Applying AQbD can overcome these problems and potentially eliminate costly human error. AQbD Practical Case: Development of a particle size measurement method for active ingredients. Handling a specific analysis case helps to clarify the application of AQbD in practice. The determination and investigation of the particle size distribution of micronized active pharmaceutical ingredients is intended to assess whether it is suitable for downstream processes and bioavailability for solid oral products. In this case, ATP is a means of determining the particle size distribution at an anchor point in the process, ensuring sufficient accuracy to ensure that the material behaves as expected. In practice, the level of precision required may exceed the requirements of the US and European Pharmacopoeia [2, 3], but for the sake of simplicity, we assume that the acceptance criteria set by the US Pharmacopoeia and the European Pharmacopoeia are sufficient. There are many techniques available for measuring particle size distribution, but for most pharmaceutical applications, laser diffraction is preferred. Therefore, we have established this AQbD example based on laser diffraction particle size measurement. Introduction to laser diffraction Due to its fast, non-destructive and easy to automate, laser diffraction has become an efficient and conventional technique for particle size measurement in modern inspection equipment. When a laser diffraction particle size analyzer is used, the particles in the sample are illuminated by a collimated laser beam. When light encounters particles, it scatters at various angles. Large particles mainly produce scattered light with small angle and high intensity, while smaller particles produce low-intensity signals at a wider distribution angle. The laser diffraction system measures the intensity of the scattered light produced by the particles as a function of angle and wavelength. A suitable light scattering model, such as Mie theory, can be used to directly calculate the particle size distribution from the measured scattered light map. Figure 2: Laser diffraction is used to determine particle size by analyzing the scattering spectra produced by the interaction of the collimated laser beam with the particles in the sample. The sample preparation work required for laser diffraction is relatively small, but the sample must be properly dispersed to make the data representative representative. In this case, it is necessary to measure the primary particle size distribution of the active pharmaceutical ingredient. This means that all agglomerates that are present must be spread out prior to measurement to ensure consistent and relevant results. The parameters used here to ensure complete dispersion are CQA, which is a variety of variables that have a direct impact on the quality of the test results. Therefore, when it comes to defining the MODR of the laser diffraction method, it is the primary goal to study dispersion in a systematic manner. Defining the scope of MODR When particle size is measured by laser diffraction, sample dispersion is involved, and a choice between dry dispersion or wet dispersion must be made. Dry dispersion is a better choice because it: Can make quick measurements; Ideal for moisture sensitive materials; Suitable for relatively large sample sizes, enabling reproducible measurements of polydisperse materials; It is environmentally friendly because it avoids the use of organic liquid dispersants. Although dry dispersion has the above advantages, it is not suitable for all types of samples. Dry dispersion requires the use of a high velocity gas stream to draw the sample. This inhalation process causes the particles to undergo tremendous shear energy and causes the particles and particles or particles to collide with the walls of the container, dispersing the various agglomerates. This method can destroy fragile materials. Because of the risks associated with nebulization, it can be dangerous to treat highly active ingredients in this manner. Therefore, some samples are more suitable for measurement methods based on wet dispersion. Figure 3: Output process of dry-dispersion key parameter evaluation results. Green indicates the interference factor in the method, orange indicates the control factor, and red indicates that the experimental study should be performed to determine the MODR. Figure 3 shows some of the CQA associated with dry micronized API powder. In dry dispersion, the air pressure applied during sample suction is used to control the energy input during the dispersion process. This shows that it is a CQA. Another consideration is the feed rate of the sample, as it determines the amount of sample passing through the venturi during the dispersion process, which in turn determines the dispersion efficiency. The concentration of the sample is also determined, which in turn has an impact on the measurement process itself. If the number or density of particles is too low, the signal to noise ratio during the measurement may be lower. Conversely, higher particle densities increase the risk of multiple scattering, ie, the interaction of light with multiple particles prior to detection, which complicates particle size calculations. Therefore, when the laser diffraction particle size measurement is performed by dry dispersion, the feed rate becomes another CQA. Therefore, based on the premise that the dry method is applied to our model samples, the above evaluation is realistic according to the CQA defined by the method. Another step – defining the MODR range is to determine how the air pressure affects the analysis. result. Experiments that provide the necessary data are often referred to as pressure titration. Figure 4 shows the results of two pressure titrations. The instrument used was the Malvern Mastersizer 3000, which has multiple modular dry dispersion units that match the particle dispersion strength to the sample. The higher two curves are the results of a standard venturi dry dispersion unit, while the lower curves are measured using a high energy venturi that provides higher dispersion energy. Figure 4: Pressure titration data for lactose formulations. A comparison of the wet (blue) and dry measurements shows a standard venturi (higher curve) when the compressed air pressure is 3 bar or a high energy venturi (lower curve) at 1 bar. The results are highly consistent. The purpose of dry dispersion is to completely disperse all agglomerates without destroying the primary particles. The results show that the particle size decreases as the pressure increases. This raises the question of how to determine if a given pressure breaks the mass as required or if it causes damage to the primary secondary particles. In contrast to the wet dispersion assay as a reference, it will help to find the answer to this question, as wet dispersion will hardly cause particle breakage. The blue color of Figure 4 shows the results of the wet dispersion measurement. The results show that the standard venturi can achieve complete dispersion of the sample particles at a pressure of about 3 bar, while the high energy venturi tube requires only about 1 bar of air pressure. These data indicate that both venturis can be used. However, by plotting the particle size of each venturi as a function of air pressure (Figure 5), it can be seen that a standard venturi is a better choice. The figure shows that a standard venturi has a wider effective working range than a high-energy venturi. Figure 5: Draw the particle size measured by each of the Venturi diffusers as a function of air pressure. The shaded area shows results consistent with the guidance in the USP <429> reference method. The standard Venturi diffuser has a wider MODR. These results indicate that with the high-energy venturi dispersion system, any subtle changes in air pressure can have a significant impact on particle size, thereby reducing the ability of the method to meet ATP. With a standard venturi, the particle size results are reasonably consistent over a pressure range of 3 to 4 bar. Therefore, standard venturis provide more stable results. Based on these data, corresponding to the appropriate air pressure, the scope of work associated with its use can be judged. Method validation The data shown in Figure 5 helps us choose the dispersion pressure to achieve the expected effective results. To ensure that the proposed method satisfies the ATP, it is important to determine that the accuracy of the results should not exceed the expected range, regardless of how the test method changes. This requires verification of the method as outlined in ICH Q2. There are two concepts that are important in determining whether a particle size determination method can achieve its goals: repeatability and reproducibility. Repeatability assessment involves repeated measurements of the same sample. Therefore, this will test the accuracy of the instrument, the consistency of the sampling and dispersion process. Reproducibility is a broader concept involving multiple operators or even multiple analysis system devices. For repeatability testing, USP [2] and EP [3] have recommended acceptance criteria. A coefficient of variation (COV) of less than 10% is considered acceptable for median (Dv50) particle size values ​​near the center of the particle size distribution or other similar particle size values. For particle size values ​​near the edge of the particle size distribution, the coefficient of variation is increased to 15%, such as Dv10 and Dv90, which represent particle sizes of 10% and 90% of the total sample volume, respectively, below this value. For samples containing particles less than 10 microns in diameter, the coefficient of variation is doubled because such a fine powder is difficult to disperse. In our case, the results acceptance criteria are implemented in accordance with the Pharmacopoeia guidelines, and the strict definition of MODR requires that any source of variation should not make the data reproducible exceed these limits. For example, control of air pressure in dispersion is one of the functions of the analyzer. As a CQA, if the air pressure requirement is controlled within ±0.1 bar, it is necessary to conduct experiments to determine the impact of this on the repeatability and reproducibility of the measured data. The potential root causes of all changes must be studied in this way. A tool that makes AQbD easier QbD and AQbD emphasize a full understanding of the process, rather than simply focusing on a range of conditions for a particular sample type. The potential advantages of this approach have been highlighted, but to get more information, a larger experiment is needed. Therefore, tools that can alleviate the burden associated with this research are naturally welcomed. Figure 6: The Mastersizer 3000 Measurement Manager presents a decentralized trend. Figure 6 shows a screenshot from the Malvern Mastersizer 3000 demonstrating the Measurement Manager tool. This is a software feature that provides real-time feedback that indicates the impact of changing analysis parameters. As part of the manual measurement control, the user can manually modify the parameters in real time; the software's SOP-player tool can also set parameters in a predefined measurement sequence. This tool provides the first step towards full automation of the method development process. In addition to assisting in the method development process, it is also required to ensure the legitimacy of the data collected to reflect the ability of the analytical method to address product changes and provide reproducible results. Here, data quality assessment tools are valuable to help guide users in defining good methods. Figure 7: Recommendations on the quality of measurement data provided by the Mastersizer 3000 software. Figure 7 shows the output from the data quality assessment tool from the Malvern Mastersizer 3000 and suggests recommendations for the measurement process (eg instrument cleanliness and calibration) and recommendations for the analysis process (eg light obtained by the instrument) The best matching effect between the scatter data and the selected optical model of the particle size distribution calculated from these data). This helps solve many of the method control problems highlighted in Figure 4. When it comes to AQbD applications, such advanced software plays an important role in greatly reducing the analytical burden associated with operations. Prospect The standardized operating procedures (SOPs) introduced about a decade ago were very groundbreaking at the time. Now, the development of analytical methods is moving beyond the simple definition of a fixed set of measurement parameters. AQbD invites analysts to build a strong MODR security analysis workspace. Working within MODR ensures that results are always consistent with the required quality standards, while being flexible enough to cope with the often encountered changes. The resulting deep understanding of the MODR scoping will ensure the effective application of analytical methods throughout the product lifecycle and greatly simplifies method migration. The highlight of QbD and AQbD is to raise awareness, but it comes with the burden of undertaking a broader research task. However, instrumentation, especially the advancement of software, can be of great help when it comes to efficiently defining the MODR range. For example, real-time feedback on measurement stability and change in results, as well as the ability to automate a range of SOPs, can greatly reduce the analytical burden and make it easier for analysts to benefit from AQbD. Satin Silk Pillow Case,Solid Color Blank Pillow Cover,Luxury Silk Sofa Pillows,Decorated Cushion Covers Shenzhen Xinmingcai Clothing Co,.Ltd , https://www.xmcsz.com